Chlorination of Water–History and Practice 
by Pure Water Annie
A new instalment in Gazette Technical Writer Pure Water Annie’s Water Treatment 101 Series
Not all micro-organisms found in water are harmful to human health. In fact, most are not. There are, however, disease-causing micro-organisms that we have learned to protect ourselves against. These are called pathogens.
Pathogens in raw water from rivers, lakes and wells can be transmitted to humans who consume the water causing waterborne diseases. Today some 80% of the developing world’s illness can be attributed to waterborne pathogens and poor sanitation.
Various strategies have been used to combat waterborne illness. In our time, the most popular of these is chlorination. Chlorine has been in use for over a century and has been found to have drawbacks; it is, however, still first choice for disinfection of water supplies. We should keep in mind that nothing lasts forever; bloodletting was the physician’s main treatment strategy just a few decades ago, and now doctors have given up their bleeding tools and leaches. Chlorine won’t be around forever, but it has served us well.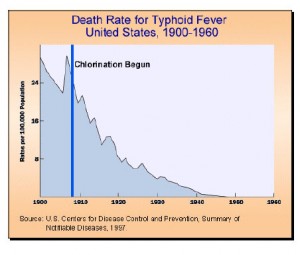
At the time when chlorine was discovered in Sweden in 1744, scientists believed that odors from water were responsible for transmitting diseases. In 1835, chlorine was being used to remove odors from water, but it wasn’t until the 1890s that people began to really comprehend that chlorine was an effective tool for disinfecting water and thus preventing waterborne disease.
Carl Wilhelm Scheele first produced chlorine in Sweden in 1744, although he did not get full credit for the discovery.
Large-scale chlorination started in Great Britain and from there was introduced in the US (1908) and Canada (1917). Today chlorination is practiced worldwide.
Chlorination’s popularity is due largely to its low cost, its relative ease of implementation, and to its broad scope of effectiveness. Chlorine inactivates pathogens by attacking their cell membrane, then entering the cell to disrupt respiration and DNA activity. It does not do this instantly, as ultraviolet light does; chlorine needs some “residence time” to be effective. Chlorine works well to control bacteria and viruses. It is not so effective, unfortunately, with protozoan cysts (giardia and cryptospridium).
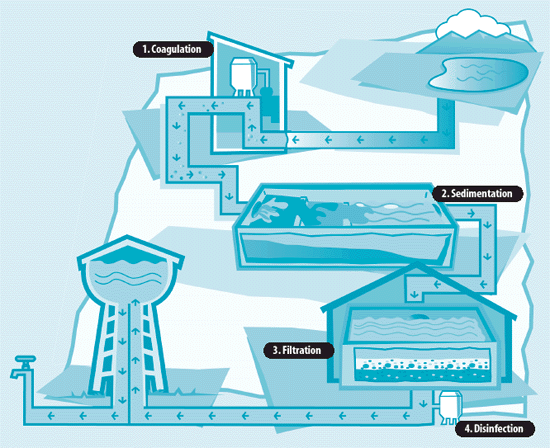
In municipal water treatment, chlorination can and often does take place at various points in the treatment process. Chlorine can be added at the very beginning of the treatment process to eliminate algae and other forms of aquatic life so that they will not interfere with the equipment in later stages of the treatment.
If chlorine is added in the next stage when water is in sedimentation tanks, where solids are allowed to settle from the raw water, it will also oxidize any iron, manganese and/or hydrogen sulfide that are present, so that they, too, can be removed in the sedimentation and filtration stages.
Disinfection with chlorine can also be done just after the sedimentation phase but before filtration. This accomplishes the same goal but does not protect equipment during sedimentation.
The most common point of chlorination, however, is at the final stage of treatment so that the chlorine can disinfect the treated water and keep it pathogen-free as it travels through the distribution system. Chlorine can also be added along the way to the final user to maintain the proper chlorine residual. Chlorinating water that is already treated is more economical because less chlorine is required after unwanted organisms have been removed by sedimentation and filtration.
Chlorine had been in use for several decades before it was learned that when chlorine combines with organics in water a large number of spin-off chemicals are formed. These unwanted by-products of chlorination are called THMs, which stands for trihalomethanes, or sometimes DBPs, which stands for disinfection by-products. Many are cancer causers and are monitored by the EPA. Many municipal water supplies are now switching from chlorine to chloramine as their disinfectant in an effort to meet EPA standards for trihalomethanes.